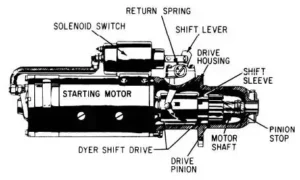
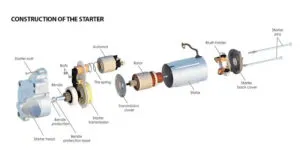
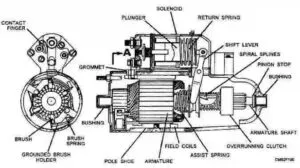
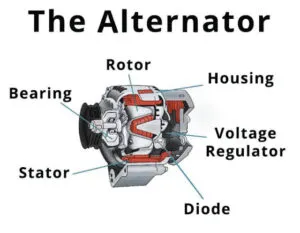
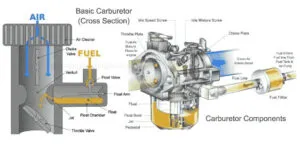
Car battery problems are a common factor for each and every vehicle owner. In this article, I will discuss 8 different types of car battery problems and how can anyone test the lead-acid battery.
Car Battery Problems
A car battery needs to be efficient to supply current to the different parts of the vehicle. It depends upon the two factors –
- Temperature.
- Rate of Discharge.
At low temperatures, the battery is less efficient and cannot supply for a long time, because chemical activities are greatly reduced and Sulphuric acid cannot work so actively on plates.
So car battery problems are always there to hamper the electricity of the vehicle. The main 8 problems are-
- Self Discharging.
- Sulphatation.
- Internal Short-Circuiting.
- Deterioration of Plates.
- Cracking of Container.
- Corrosion of Battery terminals and clamps.
- Loss of Water.
- Variations in Specific Gravity of Electrolyte.
Each and every problem is discussed below-
1. Self Discharging
If a battery is left standing for a few days, it is discharged by itself, the process is called self-discharging. New batteries usually get discharged at the rate of one percent per day of storage. The rapid self-discharging, 3% or more, is considered a serious defect of the battery.
Self-discharging of batteries takes place due to contained electrolytes, damaged separators, and long-term storage. Therefore, pure Sulphuric acid(not commercial) and distilled water(not tap water)should be used to prepare the electrolyte. Care must be taken while inspecting the battery, so that dust or dirt particles may not enter the battery.
Care must be taken while handling the battery. Battery plates or separators may not be damaged.
If a car is standing for a long period, it causes stratification of the electrolyte. The bottom layers become heavier than the top layers. In such a case local equalizing current sets up which increases the rate of self-discharging.
If a battery is self-discharging, it should be discharged to 1.2 volts per cell. This will transfer metallic impurities from negative plates to the electrolyte. Then the electrolyte is drained and the battery is washed thoroughly first with clean water and then with distilled water. Now, the fresh electrolyte is put in the battery and then it is recharged.
2. Sulphation
When a battery is discharged, the lead Sulphate forms and deposits on the plates. When it is being recharged, the Lead sulfate again converts into active materials- the lead peroxide and songy lead. However, if a battery is kept standing for a long time in a discharged condition, large lead Sulphate crystals, whitish in color, deposit on the plates, which do not convert back into the active materials of recharging. This condition is called Sulphation. The accumulation of these crystals increases the internal resistance of the cells and causes the plates to buckle or break.
The very high specific gravity of electrolytes and insufficient electrolytes are also the cause of Sulphation. Care should be taken so that sulphation may not take place. The Sulfate battery can, however, be recharged by keeping the charging rate low. It is advisable not to use heavily discharged batteries.